In this study, 2463 cases were analyzed by complete genomic profiling (CGP) for genomic alterations (GAs) and for genome wide signatures. This cohort included 479 UTUC and 1984 BUC specimens – all of these were fresh frozen paraffin embedded (FFPE) tissues. Of these samples, 77%/70% were from primary [PT] and 23%/30% metastatic tumors [MT], respectively, from unmatched patients [pts]. Tumor mutational burden (TMB) was determined on 1.1 Mbp of sequenced DNA and microsatellite instability (MSI) was determined on 114 loci. Targetable GA and signatures were assessed according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Based on pure numbers, this is a much larger study than prior studies.
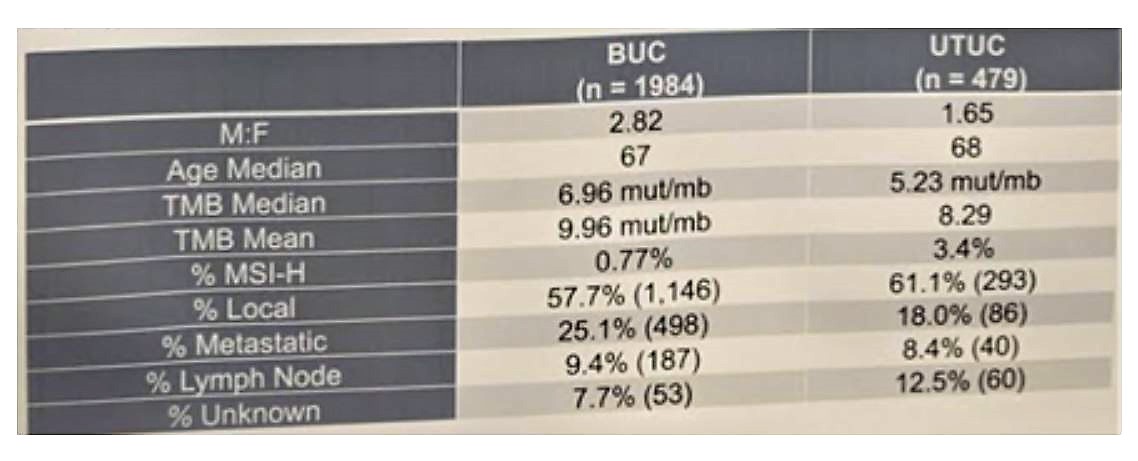
The full demographic data of the cohort is seen below:
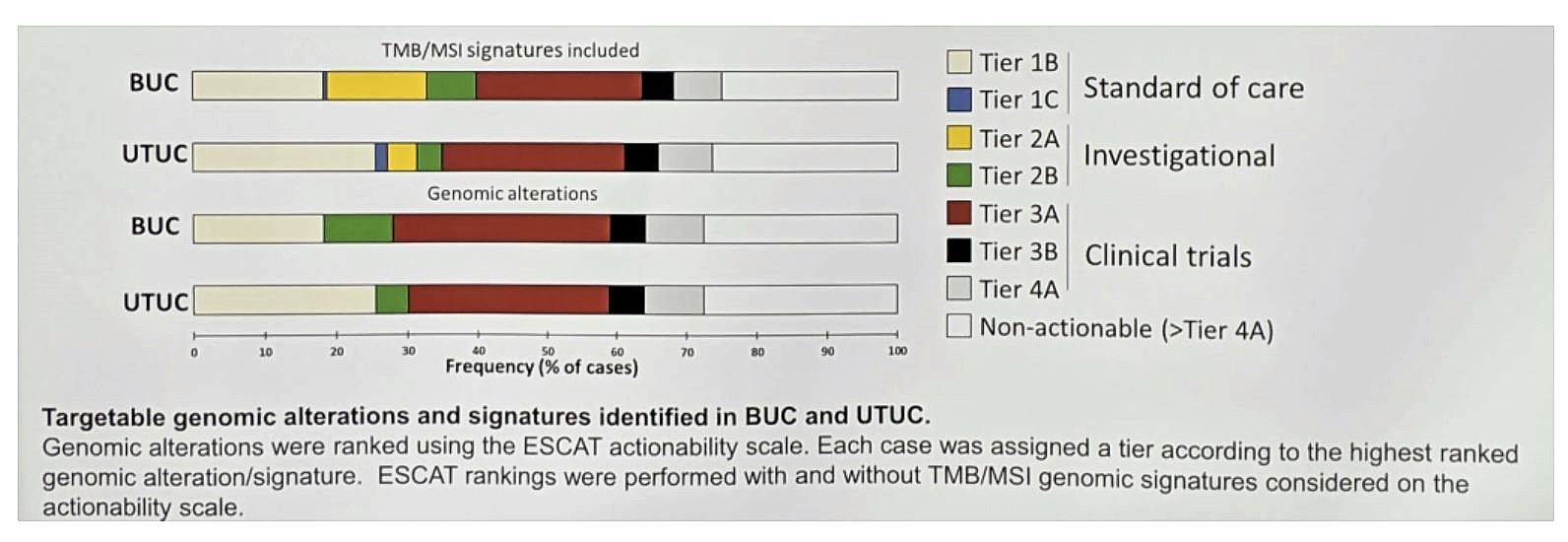
Of the entire cohort, 48% of patients harbored ≥1 tier 1-2 GA suggesting benefit from approved or investigational targeted therapies (TT). Additionally, 17% had a tier 3 GA that provides a strong rationale for clinical trial consideration.
Comparison of genomic alterations is seen below:
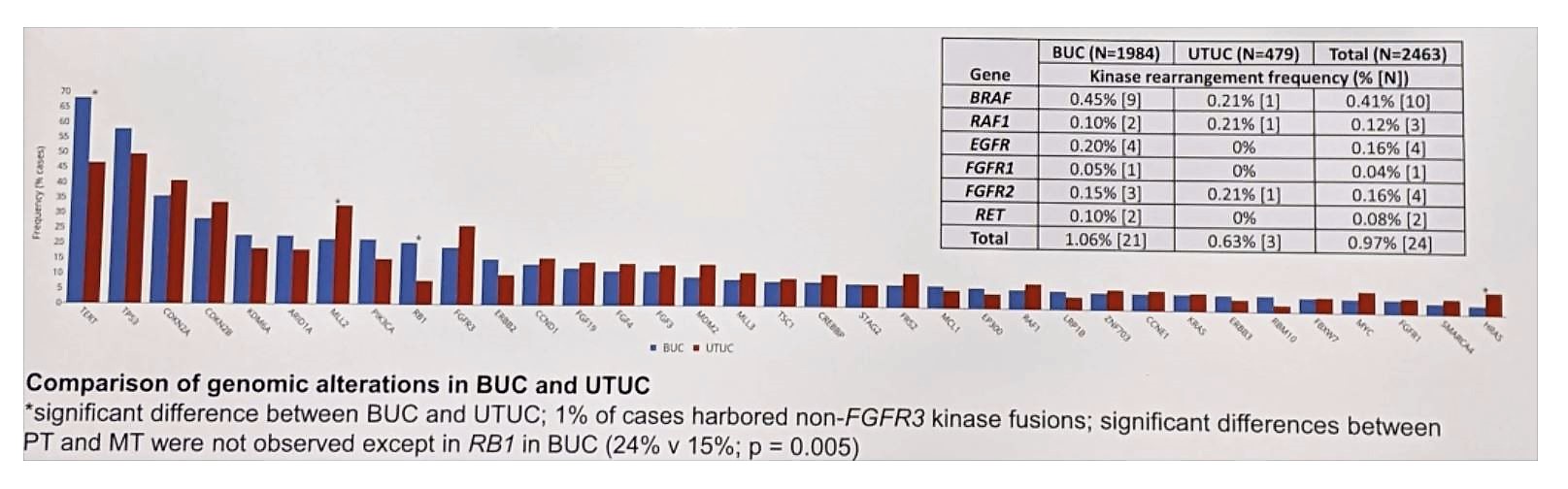
Non-FGFR3 kinase fusions were observed in 1% of patients (0.6% UTUC v 1.1% BUC), including BRAF/RAF1 fusions in 0.5%. BRAF mut/fusions were observed in 2% (49/2463) of cases and were mutually exclusive with FGFR3 GA (p = 0.002). FGFR3 GA (26% v 19%, p < 0.05) and specifically short variants (SV) (20% v 13%) were enriched in UTUC vs BUC. HRAS SV were also enriched in UTUC vs BC (7.3% v 3.0%), attributed to an enrichment in renal pelvis UC (10.1%) v ureteral UC (1.8%, p < 0.05). RB1 GA were more frequent in BUC vs UTUC (21% v 7.8% p < 0.001). All of these results are consistent with the data provided by Sfakianos et al.
When comparing UC from anatomic sites, there were no differences of TMB-H (≥20 mut/mb)/MSI-H for PT and MT specimens; however, UTUC was enriched for MSI-H (3.4%) relative to BUC (0.77%, p < 0.001, all TMB-H). Excluding MSI-H patients, UTUC has lower median TMB (4.35 mut/mb) than BUC (6.96 mut/mb).
Interestingly, they looked at concordance between tissue and cfDNA analyses in the 21 patients with matched samples, and found that if the two were done <180 days apart, concordance was much higher (67%) than if done > 180 days apart (50%); it was highest if done <90 apart (72%). In the set of patients done <180 days apart, 73% of mutations present in matched tissue samples was also detected in paired liquid biopsy samples.
Importantly, against a background of 50% actionability in UC with opportunities for immunotherapy, TT, or combinations thereof, the UTUC cohort is enriched for FGFR3 and HRAS SV relative to BUC, with the observation of HRAS mutations predominantly in UC of the renal pelvis. These results warrant further investigation into the distinct modes of oncogenesis for UC as stratified by anatomic origin. These results argue strongly for the routine incorporation of CGP prior to systemic therapy initiation in metastatic UC, as the same standard therapies are not appropriate for all patients.
Presented by: Andrea Necchi, MD, Fondazione IRCC Istituto Nazionale dei Tumori, Milan, Italy
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @JEFFUrology) at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References: