The presented study which is the PRINCIPAL study (NCT01649778), is the largest prospective real-world study of pazopanib in patients with RCC. Its main goal was to evaluate real world effectiveness and safety of pazopanib in patients with advanced and/or metastatic RCC.
All patients aged 18 and above with advanced/metastatic clear cell RCC who began treatment with pazopanib within 30 days were eligible for this trial (Figure 1).
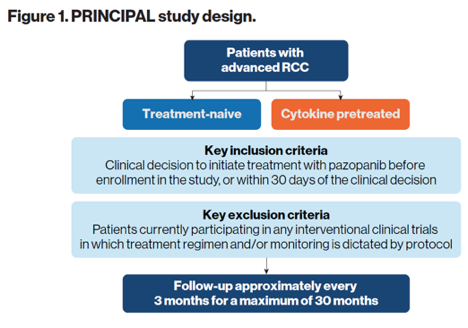
According to the study protocol, patients were followed every 3 months for a duration of 30 months, or until death, withdrawal of consent, or loss to follow-up.
The primary objective of the study was to evaluate progression free survival (PFS), overall survival (OS), and overall response rate (ORR) defined as either complete response (CR) or partial response (PR). Additional outcomes evaluated included relative dose intensity(RDI), health related quality of life, and adverse events.
Overall, 657 patients were enrolled, of which 507 (76.3%) completed the study, including 217 patients (33%) with more than 30 months of follow-up, and 284 (43.2%) patients who died during the study. Almost 10% of patients were lost to follow-up and 8.5% withdrew their consent. Table 1 demonstrates the baseline demographics and disease characteristics of the enrolled patients.

The results demonstrated that the median PFS was 10.3 months (95% CI 9.2-12) (Figure 2), and the median OS was 29.9 months (95% CI 24.7-not reached) (Figure 3).

The ORR was 30.3%, the median duration of response (CR/PR) was 11 months (95% CI 8.6-14.6), and the median time to response (CR/PR) was 3 months (95% CI 2.9-3.1). Further subgroup analyses suggested that there were poorer outcomes in patients with ECOG performance status of above 2, poor MSKCC or IMDC risk grouping, no prior nephrectomy, and cytokine pretreatment. At least one adverse event was reported by 486 (74%) patients, with the most common ones being hypertension (22.8%), diarrhea (12.8%), and elevated liver function tests (7-11.1%). Subsequent therapy after pazopanib, was given to 296 patients (45.1%), with the most common one being sunitinib.
The authors concluded that this large, prospective, observational study confirmed the effectiveness and safety profile of pazopanib in patients with advanced/metastatic RCC in a real-world clinical setting. Speaking to the authors, they noted that the limitations of this study include the potential under-reporting of adverse events, due to the observational nature of the study, and the relatively low proportion of patients with more than 30 months of follow-up.
Presented by: Manuela Schmidinger, Medical University of Vienna, Vienna, Austria
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA
References:
[1] Sternberg CN et al. J Clin Oncol. 2010;28(6):1061-1068.
[2] Motzer RJ et al. N Engl J Med. 2013;369(8):722-731.
[3] Escudier B et al. J Clin Oncol. 2014;32(14):1412-1418.
Speaker: Manuela Schmidinger, Medical University of Vienna, Vienna, Austria;