FUJI is a French multi-center (42 centers) cohort study describing OS, safety, QoL (using FACT-P) and pain (using BPI-SF) among patients with mCRPC treated with cabazitaxel in a real-world setting. From September 2013 to August 2015 patients were identified retrospectively (median follow-up 18 months), and from March 2016 to March 2017 patients were enrolled prospectively (median follow-up 6 months). For the retrospective cohort, OS was the primary outcome and predictors of OS were assessed using a multivariable Cox proportional hazards model. Safety outcomes were also assessed in the retrospective cohort.
The retrospective cohort of FUJI included 401 patients with a median age of 70 years comprising with cabazitaxel use in the second line setting (18%), third line setting (39%), fourth line setting (23%), or > fourth line setting (20%), with a median cabazitaxel use of 3.4 months. Treatment before cabazitaxel included docetaxel (100%), abiraterone acetate (77%), and enzalutamide (33%). The median OS was 11.9 months [95%CI 10.1-12.9]:
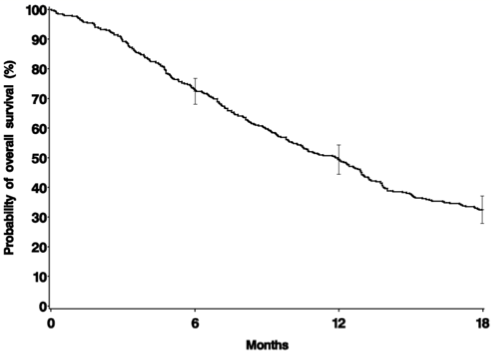
In multivariable analyses, factors associated with a shorter OS were:
- Grade ≥ 3 adverse events: HR 2.05, 95%CI 1.53-2.73
- Visceral metastases: HR 1.98, 95%CI 1.40-2.80
- Polymedication > 5 drugs: HR 1.74, 95%CI 1.23-2.45
- > 5 bone metastases: HR 1.74, 95%CI 1.20-2.53
- Disease progression during docetaxel: HR 1.69, 95%CI 1.13-2.53
- Disease progression within 3 months of last docetaxel cycle: HR 1.51, 95%CI 1.07-2.14
- ≥3 drugs such as docetaxel, abiraterone acetate, enzalutamide before cabazitaxel: HR 1.39, 95%CI 1.00-1.92
- PSA ≥ 135 ng/ml: HR 1.36, 95%CI 1.01-1.82
- ≥ 10-yr cancer history before cabazitaxel: HR 0.66, 95%CI 0.46-0.96
- ≥ 6 months from last docetaxel dose to cabazitaxel initiation: HR 0.71 95%CI 0.52-0.97
The prospective cohort of FUJI included 61 patients with a median age of 72 years, previously treated with docetaxel (98%), abiraterone acetate (61%) and enzalutamide (61%). Among 49 patients evaluable for QoL, 41% of patients experienced improved QoL, 29% maintained, and 38% deteriorated using cabazitaxel. Among 44 evaluable for pain assessment, 25% had pain decrease ≥ 1 level, 50% were stable and 25% increase ≥ 1 level.
Considering that many patients with advanced malignancies will not qualify for enrollment in clinical trials, it is important for real-world studies to review their outcomes when drugs are used outside of clinical trials. Although the median OS for patients receiving cabazitaxel as part of the FUJI cohort was lower than those in TROPIC (11.9 vs 15.1 months [1]), very few FUJI patients would have satisfied TROPIC inclusion criteria. Importantly, despite not satisfying TROPIC inclusion criteria, there were no new safety issues reported.
References:
1. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154
Presented by: Stephane Oudard, MD, PhD, Hopital Europeen Georges Pompidou, Paris, France
Co-Authors: Karim Fizazi, Florence Joly, Florence Tubach, Magali Rouyer, Stéphanie Lamarque, Estelle Guiard, Aurélie Balestra, Clémentine Lacueille, Jérémy Jové, Cécile Droz-Perroteau, Annie Fourrier-Réglat, Nicholas Moore; Institut Gustave Roussy, Villejuif, France; Centre François Baclesse, CHU Côte de Nacre, Univ. UniCaen, Caen, France; Centre de Pharmacoépidémiologie (Céphépi), Sorbonne Univ., Faculté de médecine Sorbonne Univ., AP-HP, Hôpital Pitié-Salpêtrière, INSERM, UMR 1123, CIC-P 1421, Paris, France; Bordeaux PharmacoEpi, Univ Bordeaux, Inserm CIC1401, Bordeaux, France; Bordeaux PharmacoEpi, CIC1401, Univ. Bordeaux, Bordeaux, France; Bordeaux PharmacoEpi, Univ. Bordeaux, Inserm CIC1401, Bordeaux, France; Bordeaux PharmacoEpi, Univ. Bordeaux, Inserm CIC1401 & U1219, CHU Bordeaux, Bordeaux, France
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, twitter: @zklaassen_md, at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA