For this study, the authors used data from 188 patients with urothelial carcinoma treated with durvalumab [1]. PD-L1 expression data were assessed and regression models were used to evaluate the impact of PD-L1 expression in tumor cells or tumor-infiltrating immune cells on the outcomes of OS, PFS, objective response rate (ORR), best percentage tumor change and tumor shrinkage 15 months after last subject randomization. Kaplan–Meier plots were generated to explore the impact of single biomarker and combined (tumor cells or tumor-infiltrating immune cells [% PD-L1 positive immune cells within immune cell area]) algorithms on OS.
Both tumor-infiltrating immune cells and tumor cell PD-L1 were linked to higher ORR, and PD-L1 tumor-infiltrating immune cells were associated with better survival in patients treated with durvalumab. Interestingly, PD-L1 tumor-infiltrating immune cells had a higher impact on response to durvalumab than PD-L1 tumor cell, demonstrating a significant association with OS, PFS, ORR, and tumor shrinkage. The results of several algorithms combining PD-L1 tumor cell and tumor-infiltrating immune cell cutoffs and the effect on ORR and median OS are as follows:
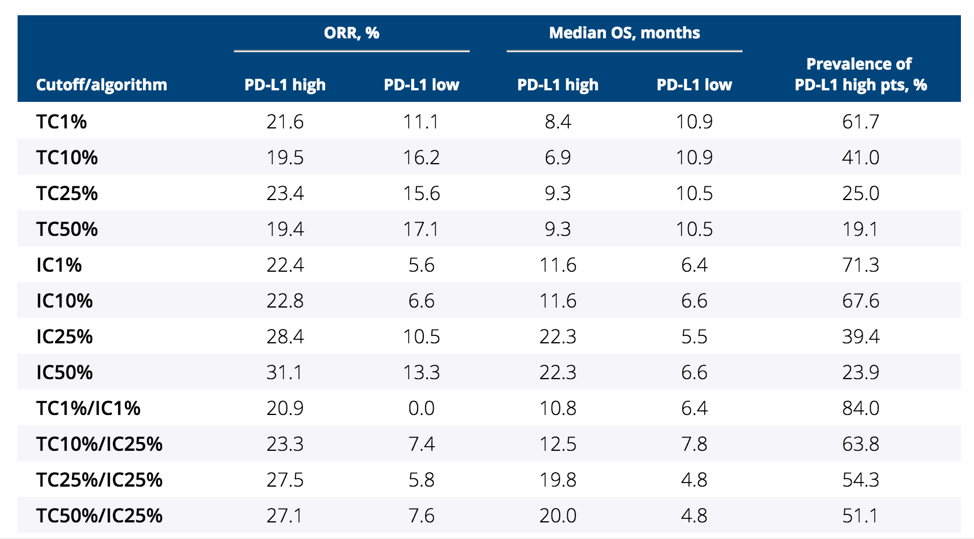
Biomarker-driven precision medicine is desired when possible. Since testing of immunotherapy agents started, PD-L1 expression has routinely been measured and results stratified by PD-L1 expression levels, however, the desired expression level and components has not been uniformly agreed. The results of this study for patients treated with durvalumab for bladder cancer suggest that a cutoff/algorithm using PD-L1 tumor cell 25%/PD-L1 tumor-infiltrating immune cell 25% (TC25%/IC25%) provides optimal predictive value based on efficacy and prevalence of the biomarker.

As Magda Zajac notes, since these results are limited to patients treated with durvalumab, additional data from randomized trials utilizing other agents are needed to confirm these findings.
References:
1. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 2017;3(9):e172411.
Presented by: Magda Zajac, AstraZeneca, Cambridge, United Kingdom
Co-Authors: Jiabu Ye, Pralay Mukhopadhyay, Xiaoping Jin, Yong Ben, Joyce Antal, Ashok Kumar Gupta, Marlon Rebelatto, J. Andrew Williams, Jill Walker; AstraZeneca, Cambridge, United Kingdom; AstraZeneca, Washington, DC; AstraZeneca, Gaithersburg, MD; DAIICHI SANKYO, Potomac, MD; AstraZeneca (currently at BioAtla, San Diego, CA, USA), Gaithersburg, MD; MedImmune (currently at G1 Therapeutics Inc, Research Triangle Park, NC, USA), Gaithersburg, MD; MedImmune, Gaithersburg, MD
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, Twitter: @zklaassen_md at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


