(UroToday.com) The third and final session of the Advanced Prostate Cancer Consensus Conference 2021 which was hosted virtually in the context of the COVID-19 pandemic focused on molecular characterization of both tissue and blood, with a focus on implications for treatment with PARP inhibitors and beyond. In this context, Dr. Andrew Armstrong discussed considerations regarding tumor molecular testing with a focus on implications for tissue-based testing as compared to liquid biopsy.
He began by emphasizing that the rationale of biopsy, regardless of approach, is to inform treatment decisions with the hope that these will improve survival, clinical benefit, and the chance of remission. However, these may also inform other decision making including family counseling, risk reduction, and clinical trial eligibility.
Dr. Armstrong then highlighted a variety of clinical guidelines recommending both somatic and germline genetic testing. While these differ somewhat, the recommendation for genetic testing in patients with metastatic castration resistant prostate cancer (mCRPC) is uniform and many of the bodies recommend testing earlier in the disease process.

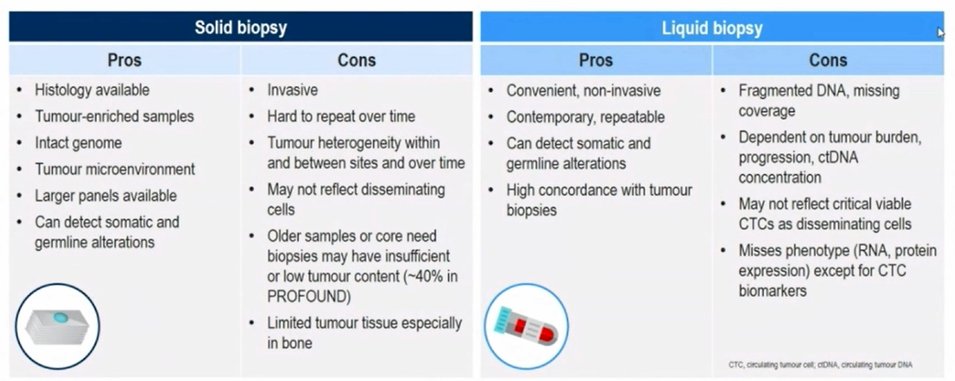
Considering how to undertake testing, Dr. Armstrong then considered the pros and cons of both solid and liquid biopsy. As summarized in the figure below, solid tumor biopsy which is the current standard of care provides the ability to assess the intact genome, the tumor microenvironment, and to assess both somatic and germline alterations. However, acquiring the necessary tissue is invasive and may be hard to repeat over time. Further, tissue biopsy may not reflect tumor heterogeneity or evolution over time. In contrast, liquid biopsy is convenient, non-invasive, and repeatable. Notably, though discussed in more detail below, liquid biopsy typically has high concordance with tumor biopsy. However, the ability to utilize liquid biopsy is dependent on tumor burden and the quality of sample obtained. Additionally, it may miss critical viable CTCs and fails to capture phenotypic characteristics.
Many (if not all) of the currently utilized ctDNA-based liquid biopsy strategies miss important tumor characteristics that may provide actionable information including genomic structural alterations, divergent genotypes, tumor phenotypes including RNA and protein expression, neoepitopes and neoantigens, host factors and other details.
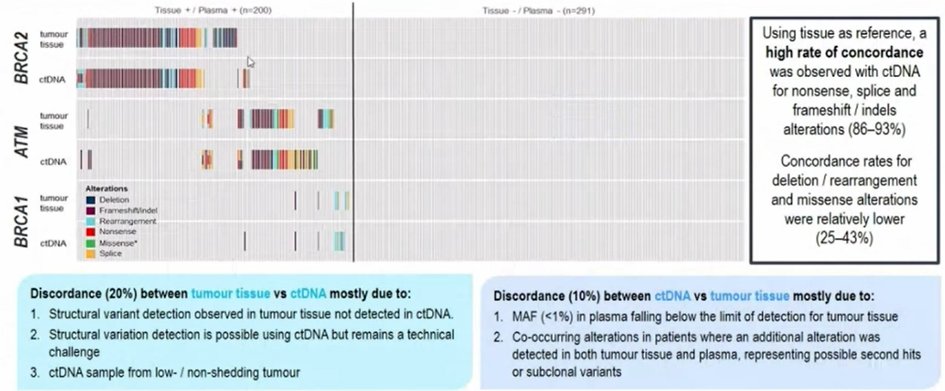
Dr. Armstrong then moved and focused on homologous recombination repair mutations. Notably, 40% of HRR carriers won’t have a relevant family history so our index of suspicion must be high. He noted that BRCA2 is the most common homologous recombination repair mutation, affecting approximately 8-9% of patients with mCRPC in PROfound, with 3.5-5.3% being germline mutations. Considering a comparison of tumor-based and ctDNA-based BRCA1, BRCA2, and ATM assessment, Dr. Armstrong highlighted that the negative agreement is high (95%) though positive agreement is somewhat lower. These mutations (along with mutations in the PI3K and Wnt pathways) are increasingly common as prostate cancer progresses as this likely reflects the fact that patients with these mutations have a more aggressive disease phenotype.
Citing again data from PROfound, he highlighted that there is generally high concordance between tumor and ctDNA testing for BRCA1, BRCA2, and ATM though some patients have tumor-tissue only while others have ctDNA only findings. These differences may be due to both technical reasons as well as disease evolution.
When considering assessment of HRR deficiency, Dr. Armstrong emphasized that the primary prostate tumor can be an important source of tissue. The concordance, overall, was 84% with clonal hematopoiesis of indeterminate potential (CHIP) contributing to 17% of the discordance observed. These data suggest that most HRR mutations are truncal and can be identified early in the carcinogenesis evolutionary tree.
As highlighted above, there are technical considerations that contribute to concordance. In particular, when the ctDNA fraction is higher, concordance rates are higher. However, discordance may be more common in specific alterations, suggesting different evolution. These include TP53, APC, RB1, and PTEN alterations.
Relying again on data from the PROfound trial, Dr. Armstrong discussed the clinical implications of different testing modalities. While the 245 patients in cohort A underwent tissue testing with FoundationOne CDx, a subset of 111 patients underwent ctDNA testing using FoundationOne Liquid CDx. When assessing the primary endpoint of radiographic progression free survival or the secondary endpoint of overall survival, the relative benefit of olaparib compared to abiraterone/enzalutamide was comparable whether patients were characterized based on tumor tissue or a ctDNA profile.
Moving on from homologous recombination repair, Dr. Armstrong then discussed the role of mismatch repair deficiency. This may affect 3-6% of patients with advanced prostate cancer who may, in turn, benefit from pembrolizumab. For the most part, these patients have mutations or rearrangements of MSH2 or MSH6. Dr. Armstrong emphasized that dMMR or microsatellite instability testing can be performed with a range of assays using a large number of probes. However, to provide reliable results, tumor content >0.1% is required.
Another potentially valuable and predictive biomarker is AR-V7 which is associated with resistance to androgen receptor pathway inhibitor resistance. This may be assayed either via an mRNA-based or a protein-based test using CTCs. This approach is the first Medicare-reimbursed and validated CTC liquid biopsy for prostate cancer. While the presence of AR-V7 is associated with poor response to androgen receptor pathway inhibitors, it has no association with response to taxane-based therapy. Thus, this predictive information may be useful in treatment selection.
Among AR-V7 negative patients, Dr. Armstrong emphasized that there are a number of other important molecular changes. In particular, he emphasized an increasing rate of androgen-receptor negative mCRPC among patients with lethal prostate cancer. Much of this is due to the development of neuroendocrine phenotypes. A neuroendocrine phenotype may be detected based on a liquid biopsy assay with a strong prognostic value in terms of progression-free survival, overall survival, and response rates.
Dr. Armstrong then highlighted common issues in ctDNA testing. The first of these is CHIP interference and confounding. CHIP presents somatic mutations that are present in blood or marrow without associated malignancy. These increase with age, smoking, and in men. Thus, these are relatively common in men with mCRPC. CHIP is independently assocaited with mortality, including cardiovascular disease. Among men with advanced prostate cancer, CHIP variants are relatively common (19%) and may involve genes which are potentially relevant for prostate cancer (eg. ATM). As a result, he suggested that orthogonal tumor testing or validation using leukocytes is reasonable for ctDNA findings.
Other issues with ctDNA testing include a low tumor burden which leads to a non-evaluable sample, missing data on ctDNA fraction, the changing disease characteristics over time, assay and probe coverage issues (such as for the androgen receptor), and an inability to capture some germline alterations.
In conclusion, Dr. Armstrong highlighted that both solid and liquid biopsy can provide valuable detection for currently clinically actionable alterations in metastatic prostate cancer. Metastatic biopsy, where feasible, allows for improved tumor content and genomic coverage as well as broader assessments of genotype and phenotype. However, liquid biopsy may be preferred where tissue is unavailable or divergent biology is suspected.
Presented by: Andrew John Armstrong, MD, Medical Oncologist, Tenured Professor of Medicine, Surgery, Pharmacology and Cancer Biology and Associate Director of the Duke Cancer Institute’s Prostate and Urologic Cancer Center
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 Advanced Prostate Cancer Consensus Conference, Saturday, October 9, 2021.