(UroToday.com) The ANZUP annual scientific meeting’s clinical trials session included an update in bladder and penile cancer provided by Dr. Dickon Hayne. Dr. Hayne notes that bladder cancer survival is poor in Australia (and has been getting worse over time) with a 5-year survival rate of 69% for males and 66% for females between 1982-1987, compared to 60% for males and 50% for females from 2006 to 2010. With regards to penile cancer, there is little centralization of penile cancer services or quaternary multi-disciplinary teams, no major clinical trials in Australia, little meaningful data, and worse outcomes for rural and indigenous men.
Dr. Hayne highlighted that there are several opportunities to improve urothelial cancer outcomes:
- Prevention: smoking
- Early diagnosis: physician education, patient education, and rapid access diagnostics
- Better treatment: peri-operatively, timely surgery, quality surgery (TURBT and cystectomy), and improved adjuvant therapy (systemic chemotherapy, immunotherapy, and intravesical treatment)
- Measure outcomes: surgical audit data, quality of life data, and stage specific recurrence free survival
Additionally, there are several ways to improve penile cancer outcomes:
- Prevention: Gardasil
- Early diagnosis: physician education, patient education, and early referrals
- Better treatment: quaternary multi-disciplinary teams, quality surgery (penile preservation and lymph node management, ie. sentinel node biopsy)
- Adjuvant therapy: systemic chemotherapy and radiotherapy, immunotherapy? Theranostics?
- Measure outcomes: surgical audit data, quality of life data, and stage specific recurrence free survival
ANZUP is currently involved in several ongoing clinical trials. First, the BCG + Mitomycin (BCGMM) trial is assessing the addition of mitomycin C to BCG as adjuvant intravesical therapy for high-risk, non-muscle-invasive bladder cancer (ANZUP 1301). Adult patients with resected, high-risk NMIBC suitable for intravesical chemotherapy are randomized to Arm A (standard) intravesical BCG induction plus maintenance or Arm B (experimental) intravesical BCG plus mitomycin C induction plus maintenance. As of September 2021, 372 patients have been recruited; the trial schema is as follows:
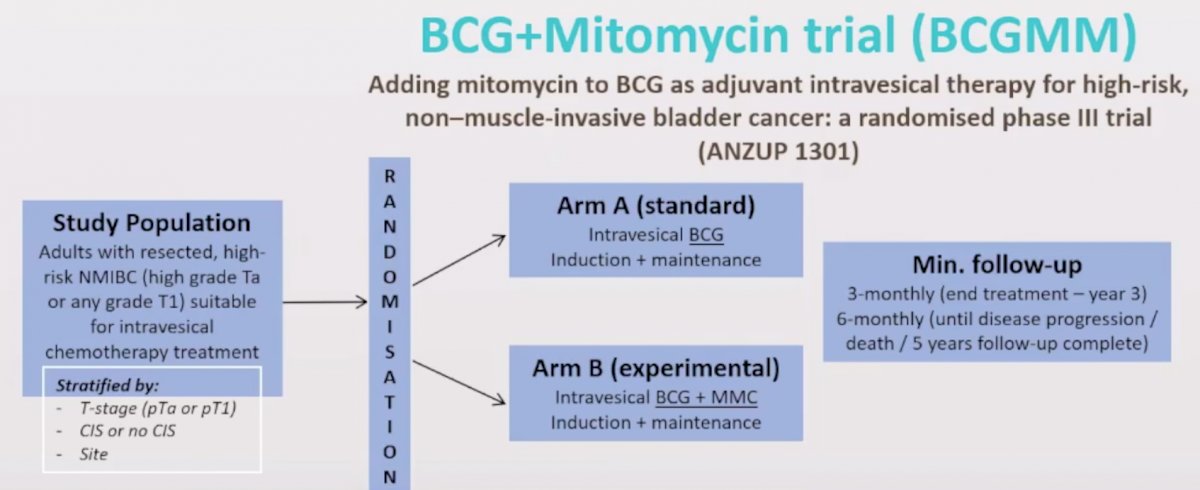
Meta-analyzed data suggest that sequential combination therapy may be superior to BCG alone, but this trial will be the first to provide high-quality data assessing the effect of combination intravesical therapy. This is the largest Australian initiated bladder cancer trial, with 372 of 500 patients accrued, including across 15 Australian sites and now recruiting in the UK. If combination chemo-immunotherapy is more effective (or equally effective) with less toxicity, it has the potential to change the paradigm for high-risk NMIBC treatment. Additionally, this trial will be a rich source of biomaterial for meaningful translational research.
The PCR-MIB trial is a multi-center single arm phase II trial evaluating the safety of adding pembrolizumab to chemoradiation in muscle-invasive bladder cancer (ANZUP 1502). Within six weeks of maximal TURBT, patients will receive chemoradiation (weekly cisplatin 35 mg/m2 x 6 doses) plus pembrolizumab (7 doses) followed by restaging cystoscopy. The trial design for PCR-MIB is as follows:
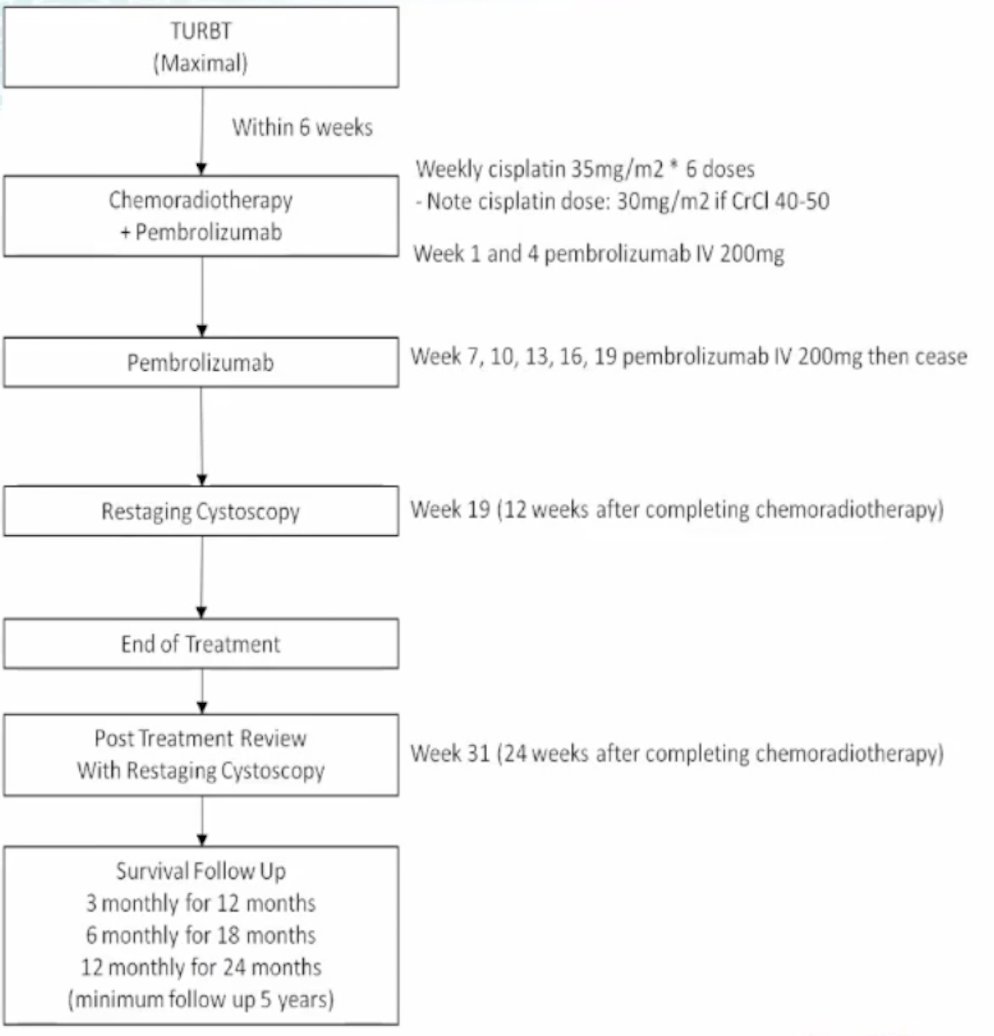
The primary outcome is feasibility measured by low rate of unacceptable toxicity and secondary outcomes include overall survival, metastatic disease-free survival, and locoregional progression free survival. The target accrual for this trial is 30 patients, with 27 patients already included.
The WATIP trial is a post-TURBT water irrigation (as a hypotonic tumor lytic agent) pilot trial with a target of 30 patients (28 accrued to date). The median age is 68 years, 23 of 28 patients are male, the median irrigation time is 3.1 hours (range 3-5 hours), with irrigation being tolerated by all patients. No adverse events have been noted, with no significant changes in serum sodium or hemoglobin. At 3 months the recurrence rate is 37%, and at 12 months the recurrence rate is 36%. This pilot trial has led to the WATER stage 1 trial, randomizing patients 1:1 to post-TURBT intravesical chemotherapy versus post-TURBT intravesical water. The target accrual for this trial is 150 patients with the following trial schema:
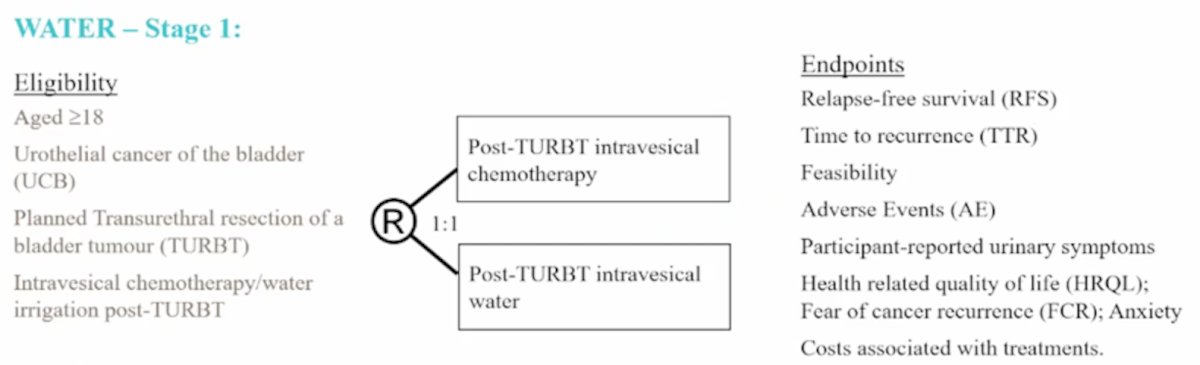
Dr. Hayne then discussed ACCEPT, the ANZUP Multi-Center Cystectomy Audit, an initiative to collect high-quality data from centers of excellence, including Fiona Stanley Hospital, Nepean Public Hospital, St. George Hospital, Hurstville Private Hospital, Royal Melbourne Hospital, Eastern Health, Royal Adelaide Hospital, Lywell McEwan Hospital, Princess Alexandra Hospital, The Canberra Hospital, and John Hunter Hospital. To date, 189 patients have been added to the registry.
The Exercyst Trial is a 20 patient (fully recruited) trial examining the efficacy and feasibility of a pre-cystectomy exercise intervention, including 12 weeks of supervised aerobic and anaerobic training. Outcomes include: recruitment and completion rates, patient safety, program adherence and compliance, length of hospital stay, 90-day complication rate, quality of life, physical functioning, and return to usual activities.
The SUBDUE-1 trial is a phase 1b open label dose-escalation trial to evaluate the tolerability, safety, and immunological efficacy of sub-urothelial durvalumab injections in adults with muscle invasive or high-risk NMIBC. To date, 9 patients have been recruited with dose escalation to 150 mg x 3 doses complete. There have been no dose limiting toxicities, all patients have proceeded to planned cystectomy, with an abstract recently submitted to GU ASCO detailing patient safety, patient reported adverse events, PROMS, and relative changes in immune cell infiltrates (CD3, CD8, CD68, and CD168). Finally, ZipUp is evaluating zirconium-girentuximab PET in urothelial cancer patients, which is a new PET scan for bladder cancer patients. ZipUp PET does not have the active agent excreted in the urine, thus allowing better visualization of the urinary tract.
Dr. Hayne notes that on the horizon is translational studies using data from the BCGMM and SUBDUE-1 studies. This will allow biomarkers predictive of response to BCG or mitomycin C, as well as mechanisms of resistance to BCG + mitomycin C that could potentially be targeted for future drug development. Results from this translational work, in combination with currently active translational work related to the SUBDUE-1 trial, may be hypothesis-generating in regard to more personalized treatment of BCG-naïve and BCG-refractory NMIBC.
Dr. Hayne concluded with several areas that ANZUP should be looking at moving forward:
- What intervention to test in high-risk NMIBC after BCGMM reads out
- Interventional trials in upper tract urothelial carcinoma: POUT-2? A surgical trial?
- Accruing to the InPACT trial for penile cancer
- Adjuvant MIBC/advanced disease, where ANZUP can impact a mostly industry-dominated disease space
Presented by: Dickon Hayne, MBBS, MD, FRCS, FRACS, Professor of Urology, The University of Western Australia Head of Urology, Fiona Stanley Hospital, Perth, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Australian and New Zealand Urogenital and Prostate (ANZUP) Cancer Trials Group Annual Scientific Meeting (ASM), Sunday, Oct 17 – Monday, Oct 18, 2021.