Session: T1 Bladder Cancer
(UroToday.com) The second session of the 8th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research (AIBCCR) Symposium was chaired by Dr Trinity Bivalacqua (University of Pennsylvania), who set the scene for T1 bladder cancer, where up to 50% of patients continue to develop disease recurrence despite BCG intravesical treatment. He highlighted the importance of preclinical modelling which can help interrogate the heterogenous cell types implicated in bladder cancer to help better understand the disease.
Dr Sima Porten (University of California San Francisco) delivered the second talk on clinical outcomes and current treatment strategies for T1 bladder cancer. She highlighted the conundrum patients and physicians face regarding bladder preservation therapy, where patients receiving intravesical BCG treatment risking disease progression versus early radical cystectomy with quality of life implications. Historic reports of the natural history of high grade (HG) T1 tumors reported a 53% risk of disease progression, 36% radical cystectomy rate and cancer specific mortality of 34% at 15 years. 1 However, more contemporary series of patients treated with adequate BCG suggest a freedom of high-grade recurrence of 74% and progression-free survival of 92% at 5 years. 2 Dr Porten highlighted several studies to support early radical cystectomy for HG T1 bladder cancer. Retrospective analysis of patients with HG pT1 bladder cancer report that deferred radical cystectomy resulted in a poorer 10-year cancer specific survival compared to patients receiving early radical cystectomy (51% vs 78%, p<0.01). 3 Further, 8% of patients with HG T1 patients were found to have lymph node positive disease at radical cystectomy, with 15% of patients upstaged to muscle-invasive bladder cancer (MIBC). 4 She stressed limitations of accurate disease staging by transurethral resection of bladder tumor (TURBT), clinical parameters, imaging and the need for better biomarkers and understanding in tumor biology. Dr Porten highlighted work from UCSF on Fibroblast activation protein inhibitor (FAPI)-PET/CT which may better assist in the identification of bladder cancer patients with occult nodal disease. FAPI which is excreted in urine limits the ability for accurate local staging, but early phase studies suggest that FAPI-PET/CT is better at identifying lymph node metastasis compared to fluorodeoxyglucose (FDG) PET/CT.
In the second talk which was on molecular and genetic aberrations on T1 bladder cancer was delivered by Dr Hikmat Al-Ahmadie (Memorial Sloan Kettering Cancer Center). He highlighted significant anatomical variation in histological landmarks where the lamina propria can be multiple folds thicker in the bladder dome compared to the trigone. 5 He pointed out limitations in pathological reporting and processing. TURBT represents a piece meal resection of tumor where lamina propria might be missed by the reporting pathologist in the sea of superficial papillary tissue. Haphazard orientation of TURBT chips by the fixation during processing can also limit the depth of tumor infiltration measurement. While sub-staging of lamina propria is recommended, there remains a lack of consensus on how this should be done. Binary classification such as focal versus extensive lamina propria invasion may provide important clinically relevant information and would allow for easy clinical adoption. Dr Al-Ahmadie concluded that genomic characterization of variant histology, tumor mutational burden and DNA damage repair genes may help compliment traditional clinical and histopathological classification.
Dr Cathy Mendelsohn (Columbia University) delivered the next talk on the investigation of luminal-basal plasticity in high grade T1 urothelial carcinoma. She presented work from her group on how chronic injury and inflammation can activate basal progenitor cells leading to bladder cancer development. Genomic classification suggested that PPAR gamma may be actively suppressed in basal/ squamous tumors which suggested that a loss of signaling may promote bladder cancer formation via MEK pathway. 6 Preclinical studies suggested that PPAR gamma mutation resulted in upregulation of NFKβ signaling resulting in squamous metaplasia. 7 She hypothesized that PPAR gamma signaling and MEK inhibition can reduce tumor growth. Her group has previously shown that utilizing mouse models, treatment with rosiglitazone (PPAR gamma agonist) and trametinib (MEK inhibitor) eradicates basal tumors in a N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) mouse model. 7
Dr John Sfakianos (Mount Sinai) then discussed the immune landscape of T1 urothelial carcinoma. He emphasized the significant disease heterogeneity in T1 bladder cancer and the upregulation of inflammatory markers in HG T1 tumors. His group utilized spatial sequencing, which allows for colocalization of gene expression, and reported that BCG naive tumors have a lower HLA-E expression and T/ NK infiltration. 8 BCG treatment then resulted in a dramatic increase in HLA-E expression. This is of relevance in BCG unresponsive tumors where despite recruitment of NK and T cells via the production of CXCL9/10/11, immune exhaustion may be responsible for treatment failure. He postulated that in patients with high levels of inflammatory cellular infiltrate, chemotherapy may be more effective than BCG. Further, priming tumors with chemotherapy prior to treatment BCG may improve BCG efficacy as second line treatment.
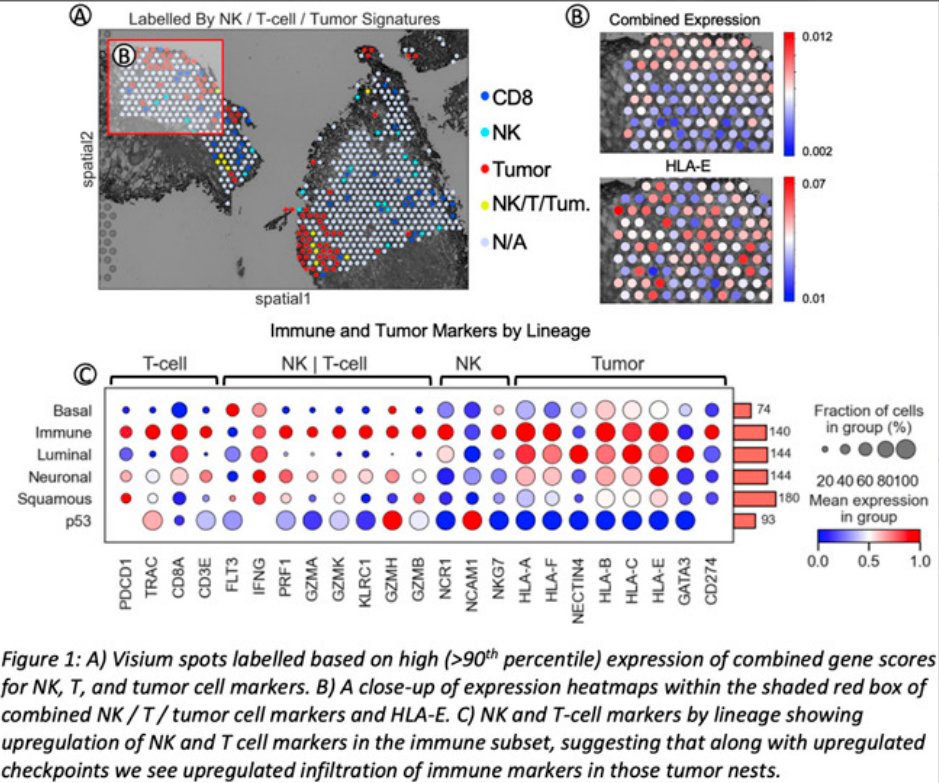
A) Visium spots labelled based on high expression of combination gene scores for NK, T and tumor cell markers. B) Magnified expression heatmaps within the shaded red box of combined NK, T or tumor cell markers and HLA-E. C) NK and T-cell markers by lineage showing upregulation of NK and T cell markers in the immune subtype, suggesting that along with upregulated checkpoints we see upregulated infiltration of immune markers in those tumor nests.
The final talk of the session was by Roger Li (Moffitt Cancer Center) on biomarkers for bladder sparing therapy in T1 bladder cancer. He highlighted the differences in predictive, prognostic and both predictive and prognostic biomarkers. Dr Li provided a comprehensive review of biomarkers in the T1 arena. He presented data on a 12-gene progression score to predict risk of NMIBC progression to MIBC which was an independent of clinical and histopathological risk factors. 9 Further work on transcriptomic profiling of T1 tumors had also identified 5 subtypes with variable response following BCG. 10 T1-Myc and T1-Early tumors had the highest risk of recurrence (58% at 24 months) with T1-TLum tumors observing the fewest recurrence (15% at 24 months) following BCG treatment. 10 Other studies he summarized include the CyPRIT cytokine urinary assay, basal versus luminal subclassification. 11, 12 He concluded that bladder cancer represents the cancer with the highest burden of copy number variation and would represent an area for future research.
Presented by: Dr Trinity Bivalacqua, MD, PhD, Director of Urologic Oncology, University of Pennsylvania; Dr Hikmat Al-Ahmadie, MD , Pathologist, Memorial Sloan Kettering Cancer Center; Dr Sima Porten, MD, MPH, Associate Professor of Urology, University of California San Francisco; Dr Cathy Mendelsohn, PhD, Professor of Urological Sciences, Pathology & Cell Biology, Columbia University; Dr John Sfakianos, MD, Assistant Professor of Urology, Mount Sinai; Roger Li, MD, Urologic oncologist, Moffitt Cancer Center,
Written by: Wei Shen Tan MBBCh, PhD, FRCS (Urol), Urologic Oncology Fellow, Department of Urology, MD Anderson Cancer Center, Twitter: @drtanws during the 2022 8th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research (AIBCCR) Friday Sept 16 – Saturday Sept 17, 2022
References:- Cookson MS, Herr HW, Zhang ZF, et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997;158(1):62-7.
- Matulay JT, Li R, Hensley PJ, et al. Contemporary Outcomes of Patients with Nonmuscle-Invasive Bladder Cancer Treated with bacillus Calmette-Guérin: Implications for Clinical Trial Design. J Urol 2021;205(6):1612-21.
- Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol 2008;53(1):146-52.
- Bruins HM, Skinner EC, Dorin RP, et al. Incidence and location of lymph node metastases in patients undergoing radical cystectomy for clinical non-muscle invasive bladder cancer: results from a prospective lymph node mapping study. Urol Oncol 2014;32(1):24.e13-9.
- Lopez-Beltran A, Cheng L. Stage T1 bladder cancer: diagnostic criteria and pitfalls. Pathology 2021;53(1):67-85.
- Liu C, Tate T, Batourina E, et al. Pparg promotes differentiation and regulates mitochondrial gene expression in bladder epithelial cells. Nat Commun 2019;10(1):4589.
- Tate T, Xiang T, Wobker SE, et al. Pparg signaling controls bladder cancer subtype and immune exclusion. Nat Commun 2021;12(1):6160.
- Ranti D, Wang Y, Daza J, et al. PD12-05 SPATIAL TRANSCRIPTOMICS PROVIDES EVIDENCE FOR ALTERNATIVE CHECKPOINT AXES IN BCG-TREATED NMIBC. Journal of Urology 2022;207(Supplement 5):e196.
- Dyrskjøt L, Reinert T, Algaba F, et al. Prognostic Impact of a 12-gene Progression Score in Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Validation Study. Eur Urol 2017;72(3):461-69.
- Robertson AG, Groeneveld CS, Jordan B, et al. Identification of Differential Tumor Subtypes of T1 Bladder Cancer. Eur Urol 2020;78(4):533-37.
- Kamat AM, Briggman J, Urbauer DL, et al. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guérin. Eur Urol 2016;69(2):197-200.
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25(2):152-65.