The clinical needs of PC can be summarized in 4 main points:
- Screening – a simple message must be conveyed to primary care physicians, who see these men more commonly than urologists
- Early detection – we need to identify patients with clinically significant PC earlier
- Reduce unnecessary repeat prostate biopsies
- Enhance risk stratification
In a large systematic review and meta-analysis published in the BMJ in 20101, all major PSA screening trials were assessed. In short, the analyses demonstrate that screening does help identify PC better. However, the main urology guidelines differ in regard to their recommendations about PSA screening. According to the AUA guidelines:
- No screening is recommended for men younger than 40
- No screening is recommended for men aged 40-55, unless risk factors are present
- In men aged 55-69 – shared decision making should be done to decide if screening is performed
- In men above 70, screening is not recommended
When assessing the age-specific upper reference limit for serum PSA, PSA 1.5 is considered in the normal range in most men below the age of 50, and in all men above the age of 50 (Figure 1).
Figure 1: Age-specific upper reference limit for serum PSA:
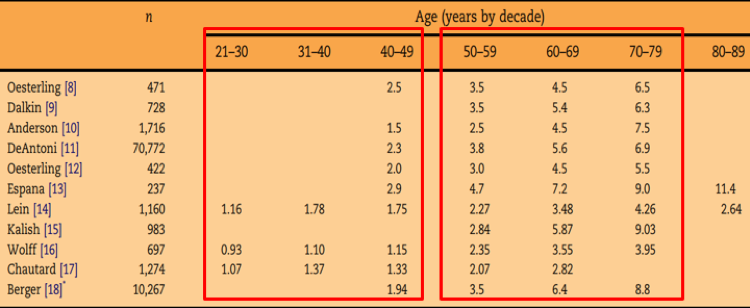
younger than 50 had a PSA less than 1.5 ng/ml, but above the age of 55, 30% had a PSA over than 1.5 ng/ml, while above the age of 60, >40% had a PSA above 1.5 ng/ml.
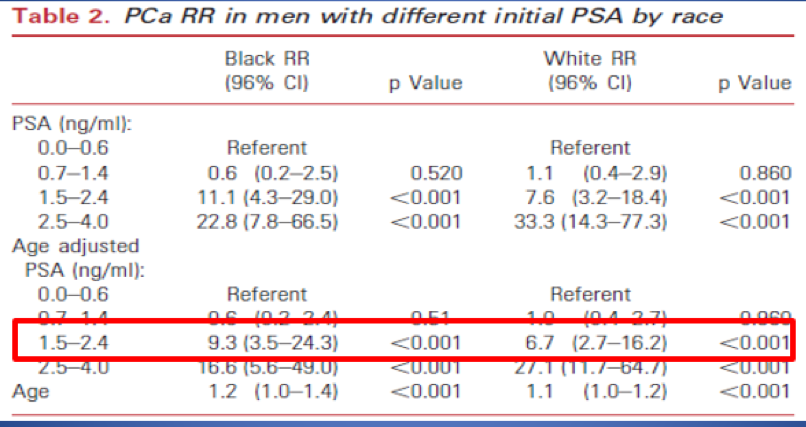
The PSA cutoff should be divided according to patient age. Below the age of 50, there is plenty of data showing that a PSA > 1.5 is correlated with higher PC Risk (Figure 2). 2,3,4 However, the era of using PSA alone to guide prostate biopsy is ending. In men older than 50, we need to use better risk assessment to identify clinically meaningful cancers. Using PSA alone, or lowering the cutoff to 1.5 ng/ml will not identify more clinically significant cancer.
Figure 2 : Increased relative risk in men <50 with PSA > 1.5 ng/ml:
The talk was summarized with some take-home messages. First, PSA screening most probably saves lives. The USPSTF analysis downplays benefits, overestimates harms and is based on far too short of a time horizon. However, overtreatment is a real public health issue and must be fixed. The answers lie in smarter, more personalized screening and better treatment decisions, not in wholesale cessation of screening, or lowering the PSA threshold. In men younger than 50, PSA >1.5 ng/ml is abnormal and warrants considering a biopsy. In men older than 50, standardized one size fits all PSA cutoff of 1.5 ng/ml can result in overdiagnosis, over-screening, over-biopsying and over treating. Incorporation of additional PC biomarkers and prostate multiparametric MRI is required in the personalization of diagnosis in the future.
Presented by: Hanan Goldberg, MD, Princess Margaret Cancer Center, Toronto, Ontario, Canada
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel
References:
1. Djulbegovic Mia, et al. BMJ 2010
2. Ping T et al. J. Urol 2010
3. Lilja H, et al. Cancer. 2011
4. Goldberg H. et al. J Urol 2018
Read the Opposing Side: A PSA Threshold of 1.5 ng/ml for Further Diagnostic Tests - FOR