There has been some advancement in technique and instrumentation, resulting in the possibility of ureteroscopic management of UTUC. This was initially reserved for patients with absolute indications for nephron preservation. According to the most recent NCCN guidelines, endoscopic treatment for UTUC is reserved for low-grade UTUC. The EAU guidelines recommend unifocal kidney-sparing management in case of a unifocal tumor, tumor size less than 2 cm, low-grade tumor, with no evidence of infiltrative lesion on CTU, with an understanding that these patients should be closely followed. According to most studies published, ureteroscopic approach manages to preserve the kidney in 60-83% of cases. However, local recurrence rates range between 60-83% of cases with progression rates between 11-15%.
When looking at the long-term follow-up (20 years) of endoscopic management of UTUC, a recurrence rate of 68% was observed after a median follow-up of 54 months (1-223 months), with a 5-year and 10-year cancer-specific survival (CSS) of 88.9% and 77.4%, respectively.1 Almost 25% of the patients underwent adjuvant topical treatment with standard Mitomycin (MMC), which has an unknown and unexplored efficacy in UTUC.
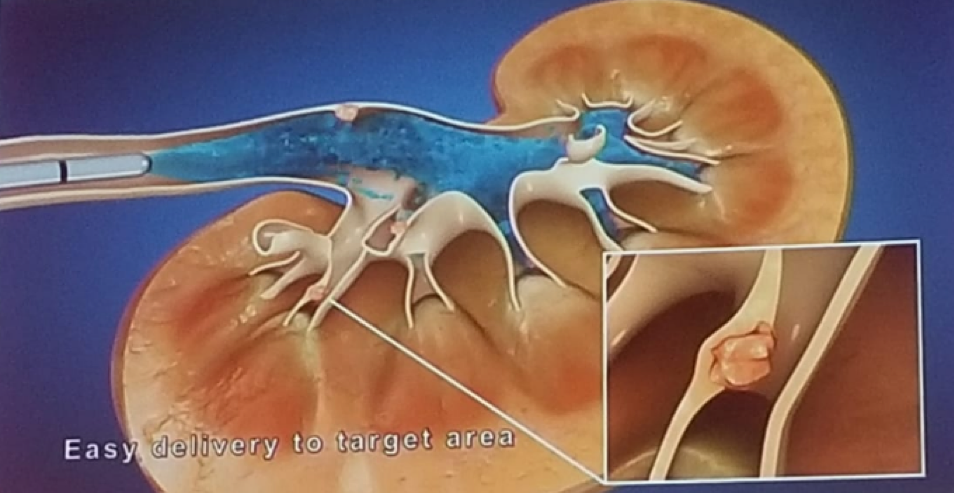
Kleinmann then moved on to discuss MitoGel, which is a novel RTGel/MMC sustained release formulation. This compound consists of both Mitomycin and can liquefy when chilled. It enables easy delivery to the target area and does not interfere with normal kidney function (Figure 1). The initial clinical experience with it in UTUC patients (based on compassionate treatment), was given in extreme cases, including localized treatment of unresectable high burden tumors. This was done in the US, Europe and Israel.
Figure 1 – MitoGel delivery:
Kleinmann went on and presented this initial clinical experience, which included 22 patients enrolled, of which 14 completed treatment, and 13 evaluated. In the intention to treat analysis, 18 patients with confirmed low-grade UTUC disease were given the treatment with a complete response rate of 44%. Overall, 8/22 patients responded completely, 3/22 had endured recurrence, 4/22 remained with complete response > 12 months, and one patient was lost to follow-up.
Kleinmann then described the OLYMPUS (Optimized Delivery of Mitomycin for Primary UTUC Study) trial. In this trial, a total of 34 patients with a tumor larger than 5 mm, were given 6 instillations of MitoGel. The interim results demonstrate that out of the 34 patients recruited, almost 40% had an unreachable tumor at baseline, and 56% had multiple tumors at baseline. Overall, 13/13 patients that reached 3 months follow-up had a durable complete response. The 4/4 patients that reached 6 months follow-up had a durable response, and lastly, 1 patient that reached 9 months of follow-up, had a durable complete response. The authors concluded that this experimental treatment is feasible and safe with the majority of adverse effects reported as mild or moderate and had resolved.
Kleinmann concluded his talk by stating that low-grade non-invasive UTUC disease can be ablated using an RT-Gel technology, potentially resulting in less surgical intervention and RNUs. This pivotal study continues to enroll patients.
References:
1. Cutress M.L et al. Long term endoscopic management of upper tract urothelial carcinoma20 year single center experience. BJU Int. 2012 Dec;110(11):1608-17. doi: 10.1111/j.1464-410X.2012.11169.x. Epub 2012 May 7.
*MitoGel is sustained-release mitomycin C hydrogel formulation - A sustained-release (SR) hydrogel polymer-based formulation containing the antineoplastic antibiotic mitomycin C (MMC), with potential antineoplastic activity. Upon local administration of the SR MMC hydrogel formulation to the upper urinary tract via a ureteral catheter, the gel solidifies and deposits MMC locally to prevent the excretion of this chemotherapeutic agent via urinary flow. In turn, MMC alkylates DNA and produces interstrand DNA cross-links, thereby inhibiting DNA synthesis. Due to its reverse thermal-gelation properties, this gel is able to stay in a liquid state at cold temperatures and solidifies at body temperature. This allows for increased accumulation of MMC locally in the upper urinary tract which leads to increased efficacy compared to standard intravesical delivery of MMC for upper tract urothelial carcinoma (UTUC).
Presented by: Nir Kleinmann, MD, Tel-Hashomer Hospital, Israel
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 FOIU 4th Friends of Israel Urological Symposium, July 3-5. 2018, Tel-Aviv, Israel