Barcelona, Spain (UroToday.com) Multiple therapeutic options have been approved for the treatment of men with metastatic castration-resistant prostate cancer (mCRPC), including the second-generation anti-androgens abiraterone and enzalutamide, and chemotherapy with docetaxel or cabazitaxel. However, the appropriate order in which to stagger these therapies is not uniformly clear. With regards to anti-androgens, there is the suggestion that enzalutamide may be effective after progression on abiraterone and less suggestion that abiraterone is effective after enzalutamide. Additionally, while many patients do respond to newer anti-androgen therapies, a subset of patients progress within a year or less, representing a more aggressive disease phenotype that may benefit from chemotherapy rather than another anti-androgen.
To address these issues, Dr. Ronald De Wit presented results from the CARD study, an open label investigation of men with mCRPC who progressed within 12 months on either abiraterone or enzalutamide and were then randomized to either cabazitaxel or the other anti-androgen therapy. Other key inclusion criteria included at least 3 prior cycles of docetaxel, castration levels of testosterone, and of note, docetaxel, and abiraterone in the hormone-sensitive setting were allowed. Importantly, the European dose of 25 mg/m2 of cabazitaxel with G-CSF prophylaxis was given, which is in contrast to the equally effective FDA approved dose of 20 mg/m2. The primary endpoint was radiographic progression-free survival (PFS). Secondary endpoints were overall survival, PFS and tumor response. Other secondary data outcomes such as pain response and quality of life data were not reported today.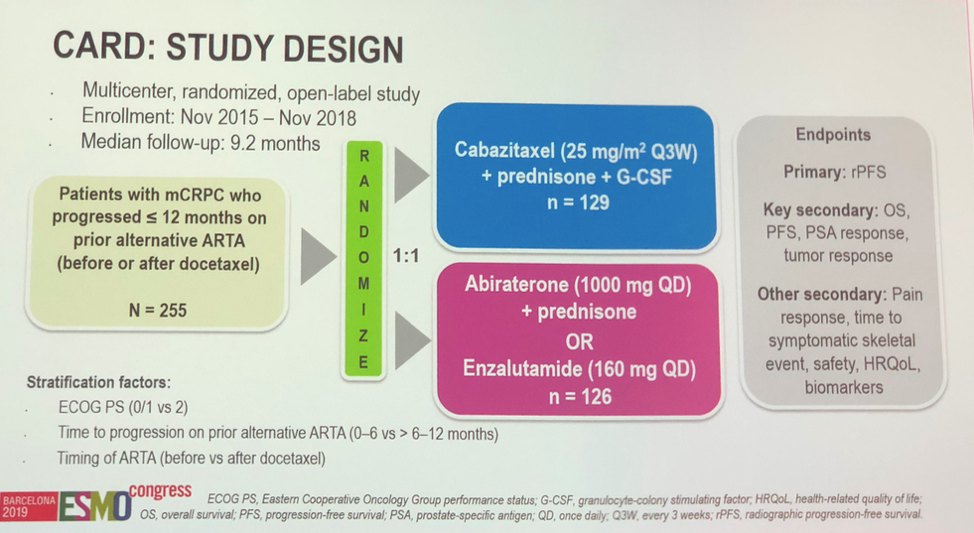
Patient cohorts were well-balanced between arms and representative of a real-world patient population.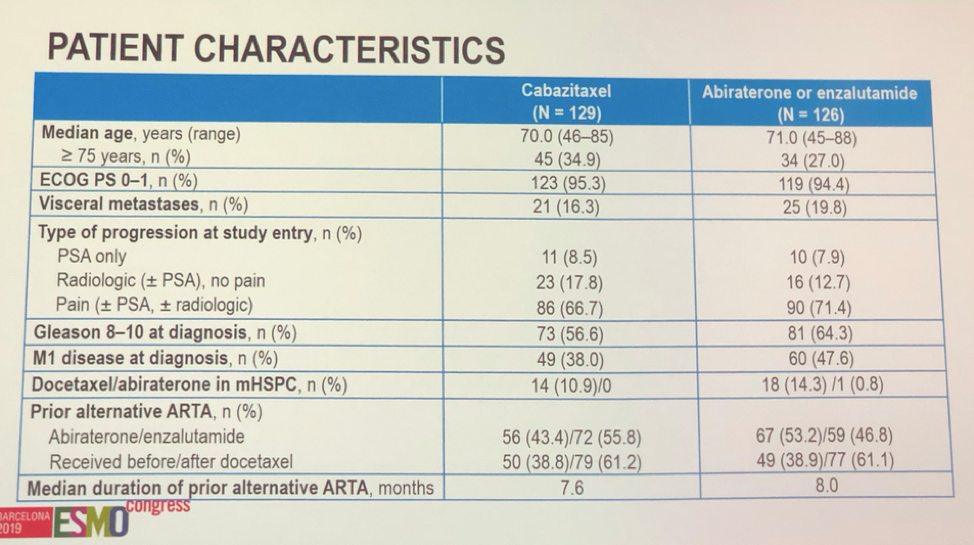
A median of 22 weeks or 7 cycles of cabazitaxel were administered, as opposed to 12.5 weeks of alternative anti-androgen. 20% of patients in the cabazitaxel arm discontinued treatment due to treatment side effects, which is attributed to the total number of cumulative chemotherapy doses. More patients discontinued therapy in the alternative anti-androgen arm due to disease progression.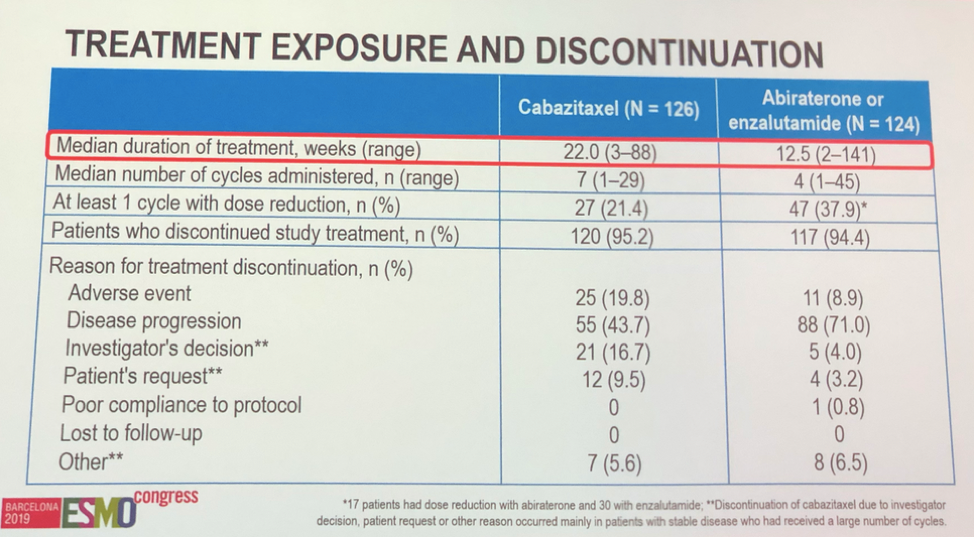
Side effects were generally balanced between groups. Febrile neutropenia was only noted in 3.2% of patients.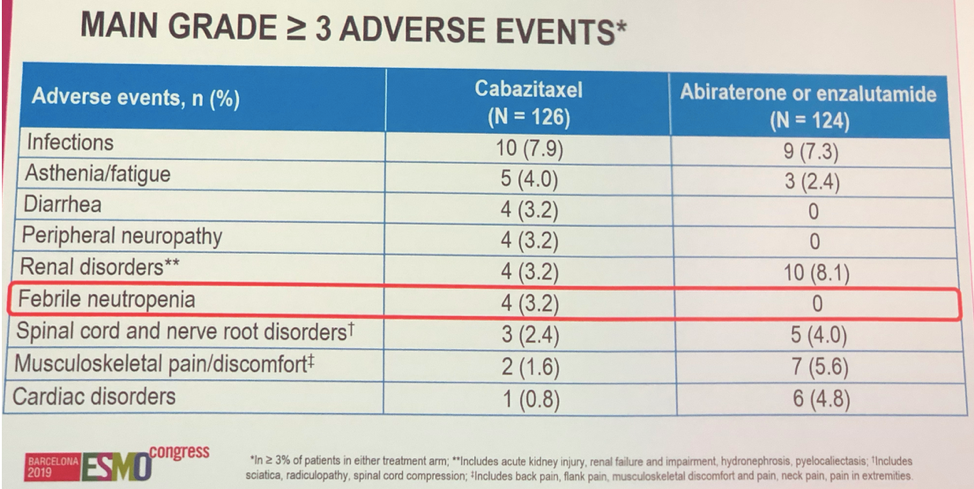
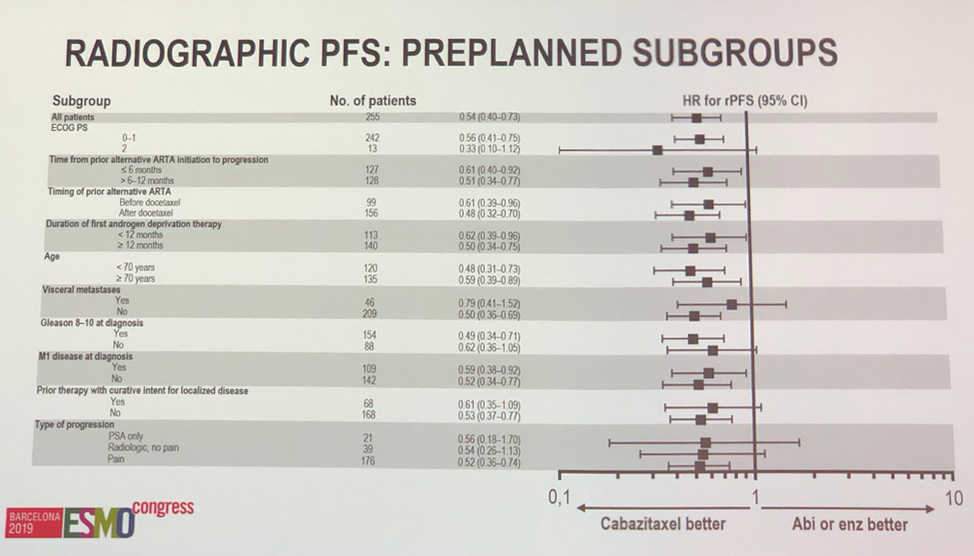
With regards to the primary outcome, cabazitaxel treatment offered statistically significant radiographic progression free survival benefit over alternative anti-androgen therapy (HR 0.54, P < 0.001). Pre-planned subgroup analysis showed statistically significant benefit with cabazitaxel in almost all sub-groups, and if not significant, data suggested a trend towards benefit with cabazitaxel.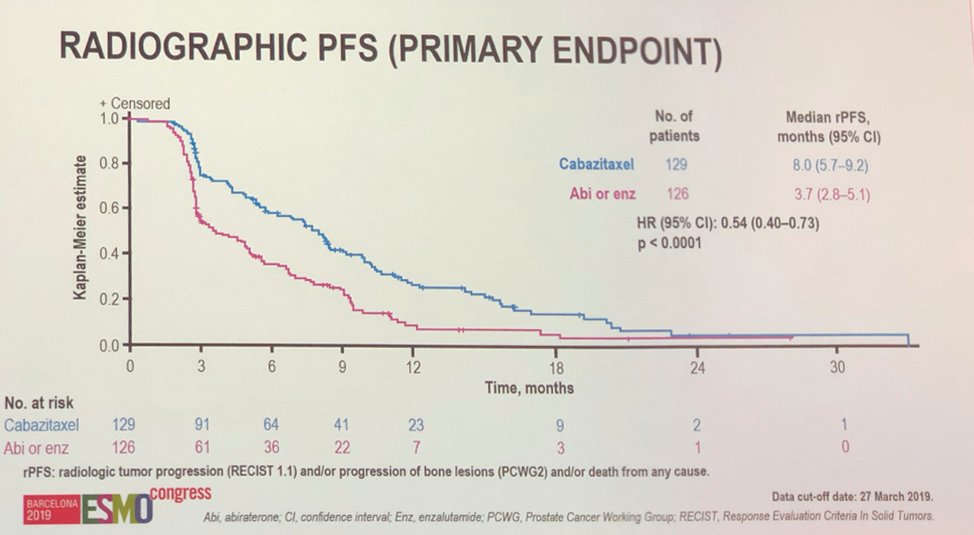
Overall survival data also suggests a benefit for cabazitaxel. A benefit for other secondary outcomes such as PSA, pain response and tumor size were also seen with cabazitaxel.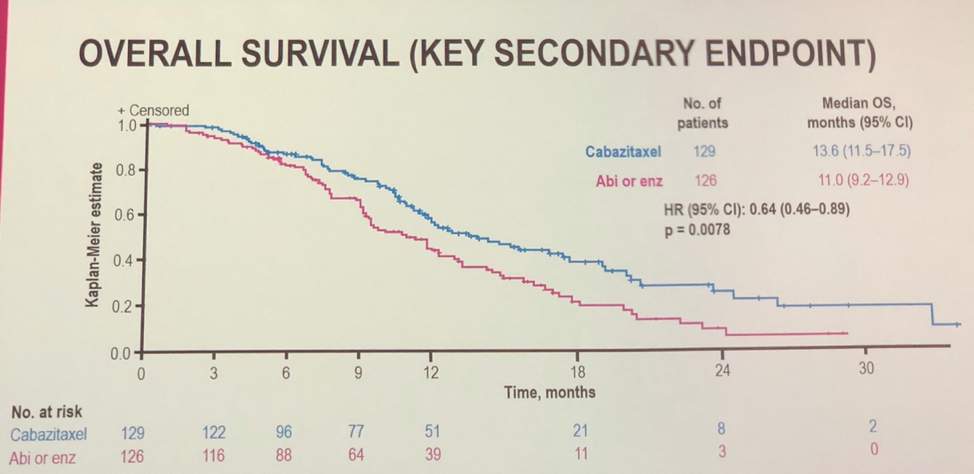
Importantly, in a post-hoc analysis, the authors showed that regardless of which anti-androgen is administered first, the second anti-androgen has minimal efficacy, especially relative to cabazitaxel.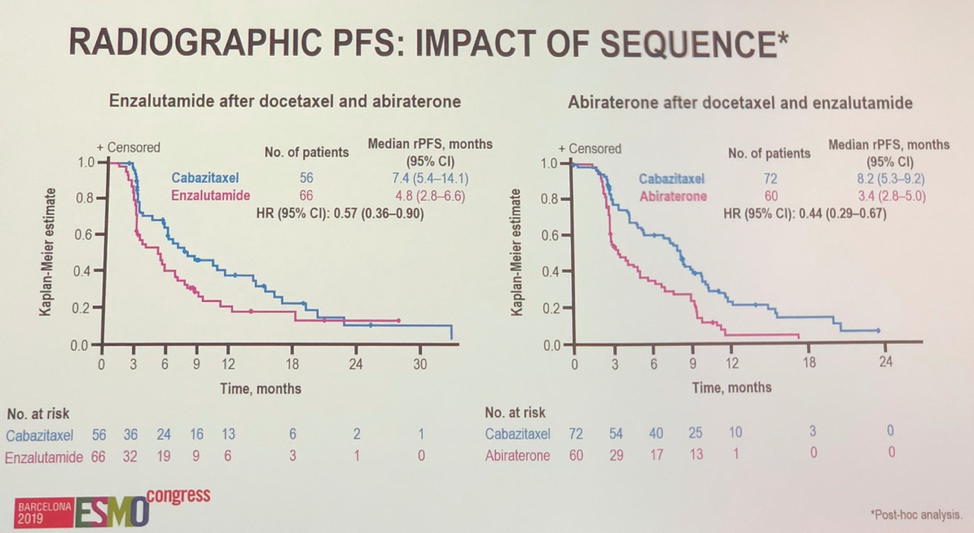
Together, this data suggests that cabazitaxel should be a standard third line of care for patients who have received one of either abiraterone or enzalutamide and docetaxel chemotherapy.
Following Dr. De Wit’s presentation, the CARD trial was published in The New England Journal of Medicine
Reference:
de Wit, Ronald et al. 2019. "Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer". New England Journal of Medicine. Massachusetts Medical Society. doi:10.1056/nejmoa1911206.
Clinical trial identification: NCT02485691
Presented by: Ronald De Wit, MD, Ph.D., Medical Oncologist at the Erasmus Medical Center, Rotterdam, Netherlands
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain
Further Related Content:
ESMO 2019: Invited Discussant (LBA13) - The CARD Trial, A Randomized, Open-Label Study of Cabazitaxel vs Abiraterone or Enzalutamide in mCRPC


